Search any question & find its solution
Question:
Answered & Verified by Expert
A benzene derivative did not produce white precipitate with the ammonical silver nitrate solution but decolorised the cold dilute alkaline $\mathrm{KMnO}_4$ solution. The compound is
Options:
Solution:
2998 Upvotes
Verified Answer
The correct answer is:
$\mathrm{C}_8 \mathrm{H}_8$
$\mathrm{C}_8 \mathrm{H}_8$ is
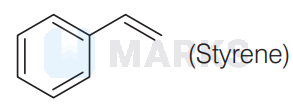
It is a derivative of benzene and decolorised the cold alkaline $\mathrm{KMnO}_4$ solution.
The reaction occurs as follows :
$\mathrm{C}_8 \mathrm{H}_8+2 \mathrm{KMnO}_4 \longrightarrow \mathrm{KC}_8 \mathrm{H}_5 \mathrm{O}_2$ $+2 \mathrm{MnO}_2+\mathrm{KOH}+\mathrm{H}_2 \mathrm{O}$

It is a derivative of benzene and decolorised the cold alkaline $\mathrm{KMnO}_4$ solution.
The reaction occurs as follows :
$\mathrm{C}_8 \mathrm{H}_8+2 \mathrm{KMnO}_4 \longrightarrow \mathrm{KC}_8 \mathrm{H}_5 \mathrm{O}_2$ $+2 \mathrm{MnO}_2+\mathrm{KOH}+\mathrm{H}_2 \mathrm{O}$
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.