Search any question & find its solution
Question:
Answered & Verified by Expert
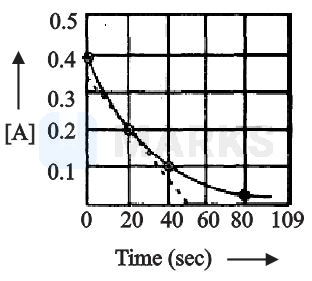
A certain reaction follows the given concentration (molarity)-time graph. Which of the following statement is true?
Options:

Solution:
2301 Upvotes
Verified Answer
The correct answer is:
The rate for this reaction at 40 second will be approximately
Time required for concentration to change from to is same as the time required for the concentration to change from to , it means is independent of initial concentration, i.e. it is a first order reaction.
And from the graph
At second
And from the graph
At second
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.