Search any question & find its solution
Question:
Answered & Verified by Expert
A constant amount of an ideal gas undergoes the cyclic process ABCA in the PV diagram shown below
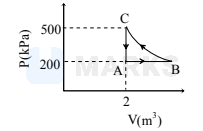
The path $\mathrm{BC}$ is an isothermal. The work done by the gas during one complete cycle, beginning and ending at A, is nearly-
Options:

The path $\mathrm{BC}$ is an isothermal. The work done by the gas during one complete cycle, beginning and ending at A, is nearly-
Solution:
1503 Upvotes
Verified Answer
The correct answer is:
$-300 \mathrm{~kJ}$
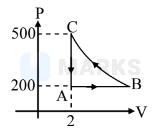
$\begin{array}{l}
\mathrm{W}_{\mathrm{C} \rightarrow \mathrm{A}}=0 \\
\text { Process } \mathrm{BC} \quad \mathrm{T}=\text { Constant } \\
\mathrm{P}_{\mathrm{C}} \mathrm{V}_{\mathrm{C}}=\mathrm{P}_{\mathrm{B}} \mathrm{V}_{\mathrm{B}} \\
500 \times 2=200 \times \mathrm{V}_{\mathrm{B}} \\
\mathrm{V}_{\mathrm{B}}=5 \mathrm{~m}^{3} \\
\mathrm{~W}_{\mathrm{A} \rightarrow \mathrm{B}}=200\left[\mathrm{~V}_{\mathrm{B}}-\mathrm{V}_{\mathrm{A}}\right]=200[5-2] \\
=600 \\
\mathrm{~W}_{\mathrm{B} \rightarrow \mathrm{C}}>\mathrm{W}_{\mathrm{A} \rightarrow \mathrm{B}}
\end{array}$
$\therefore$ Net work done is $-\mathrm{ve}$
$\begin{array}{l}
\because \mathrm{W}_{\mathrm{B} \rightarrow \mathrm{C}} < 1200 \mathrm{KJ} \\
\therefore \text { Total } \mathrm{W} < -600 \mathrm{KJ}
\end{array}$
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.