Search any question & find its solution
Question:
Answered & Verified by Expert
A diatomic ideal gas undergoes a thermodynamic change according to the diagram shown in the figure. The total heat given to the gas is nearly (use )
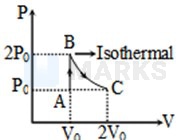
Options:

Solution:
1327 Upvotes
Verified Answer
The correct answer is:
, where
According to the first law of thermodynamics
Where
therefore
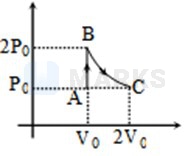
Process
According to the first law of thermodynamics
Where
therefore

Process
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.