Search any question & find its solution
Question:
Answered & Verified by Expert
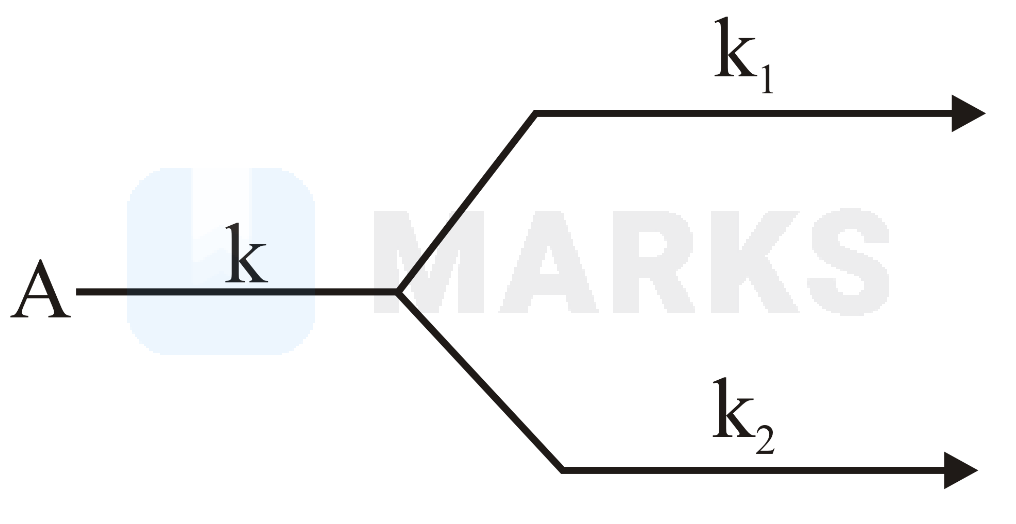
A molecule undergoes two independent first order reactions whose respective half lives are and . If both the reactions are occurring then the time taken for the consumption of the reactant is ______ . (Nearest integer)
Solution:
1327 Upvotes
Verified Answer
The correct answer is:
2
For parallel reaction
When adding the rate constants of the two reactions, we need to use the formula for combining rate constants of two reactions in parallel, which is:
where and are the rate constants of the individual reactions.
In this case, the half-lives of the two reactions are min and min, respectively. The rate constants of the two reactions can be calculated using the formula for half-life of a first-order reaction:
Net
Hence time taken for consumption of reactant will be close to .
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.