Search any question & find its solution
Question:
Answered & Verified by Expert
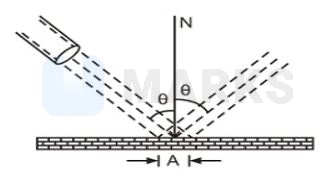
A nozzle throws a stream of gas against a wall with a velocity much larger than the thermal agitation of the molecules. After collision of the molecules with wall the magnitude of their velocity remains same. Also assume that the force exerted on the wall by the molecules is perpendicular to wall. (This is not strictly true for a rough wall.) Find the pressure exerted on the wall. number of molecules per unit volume, mass of a gas molecule
Options:

Solution:
1260 Upvotes
Verified Answer
The correct answer is:
Since, the molecules rebound from the wall, the component of velocity perpendicular to wall is reversed, while its velocity parallel to wall does not change. The change in velocity of molecules is parallel to normal N. The magnitude of charge is
The change in momentum of a molecule is
in the direction of normal N. Let n be the number of molecules per unit volume.The number of molecules arriving at an area A of the wall per unit time is the number in a slanted cylinder whose length is equal to the velocity v and whose cross-section is
Number of molecules .
Each molecule suffers a change of momentum . Change of momentum of the stream of gas in a direction perpendicular to wall is equal to .
Hence, force exerted by stream of gas on the wall.
This is also the force exerted by gas molecules on the wall.
The change in momentum of a molecule is
in the direction of normal N. Let n be the number of molecules per unit volume.The number of molecules arriving at an area A of the wall per unit time is the number in a slanted cylinder whose length is equal to the velocity v and whose cross-section is
Number of molecules .
Each molecule suffers a change of momentum . Change of momentum of the stream of gas in a direction perpendicular to wall is equal to .
Hence, force exerted by stream of gas on the wall.
This is also the force exerted by gas molecules on the wall.
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.