Search any question & find its solution
Question:
Answered & Verified by Expert
A reaction of cobalt chloride and ethylenediamine in a mole ratio generates two isomeric products (violet-coloured) and (green-coloured). can show optical activity, but, is optically inactive. What type of isomers do and represent?
Options:
Solution:
2044 Upvotes
Verified Answer
The correct answer is:
Geometrical isomers
The coordination number of cobalt is .
The mole ratio of and is .
Hence,
The complex formed shows geometrical isomerism.
The complex exists as cis and trans isomers among which the trans isomer is optically inactive.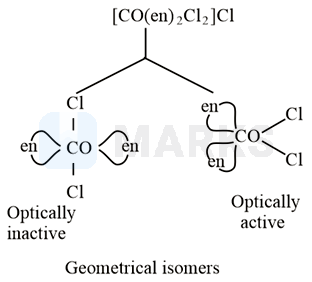
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.