Search any question & find its solution
Question:
Answered & Verified by Expert
A Substance undergoes first order decomposition. The decomposition follows two parallel first order reactions as
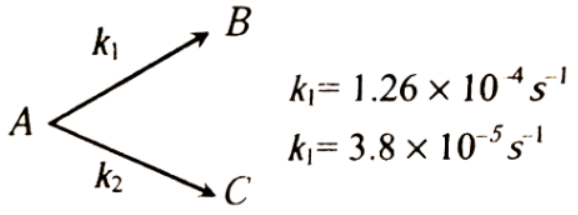
The percentage distribution of \(B\) and \(C\) are
Options:
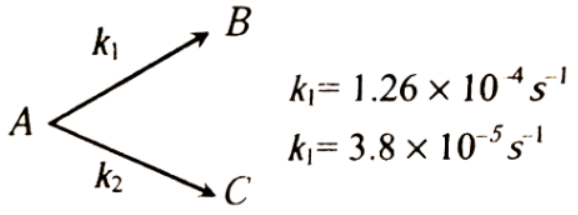
The percentage distribution of \(B\) and \(C\) are
Solution:
1995 Upvotes
Verified Answer
The correct answer is:
\(76.83 \% \mathrm{~B}\) and \(23.17 \% \mathrm{C}\)
\(\begin{aligned} & \text { % distribution of } \mathrm{B}=\frac{k_1}{k_1+k_2} \times 100 \\ & =\frac{1.26 \times 10^{-4}}{1.26 \times 10^{-4}+3.8 \times 10^{-4}} \times 100 \\ & \mathrm{~B} \%=76.83 \% \\ & \text { % Distribution of } \mathrm{C}=\frac{k_2}{k_1+k_2} \times 100 \\ & =\frac{3.8 \times 10^{-4}}{1.26 \times 10^{-4}+3.8 \times 10^{-4}} \times 100 \\ & \mathrm{C} \%=23.17 \%\end{aligned}\)
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.