Search any question & find its solution
Question:
Answered & Verified by Expert
Among the following compounds, which compound is polar as well as exhibit $s p^2$-hybridisation by the central atom
Options:
Solution:
1094 Upvotes
Verified Answer
The correct answer is:
$\mathrm{H}_2 \mathrm{CO}_3$
(i) $\mathrm{SiF}_4$ and $\mathrm{HClO}_3$ has $s p^3$ - hybridisation.
(ii) $\mathrm{BF}_3$ and $\mathrm{H}_2 \mathrm{CO}_3$ has $s p^2$-hybridisation but $\mathrm{BF}_3$ is non-polar.
$\therefore$ Correct answer is $\mathrm{H}_2 \mathrm{CO}_3$.
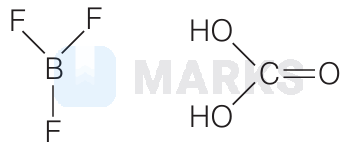
(ii) $\mathrm{BF}_3$ and $\mathrm{H}_2 \mathrm{CO}_3$ has $s p^2$-hybridisation but $\mathrm{BF}_3$ is non-polar.
$\therefore$ Correct answer is $\mathrm{H}_2 \mathrm{CO}_3$.

Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.