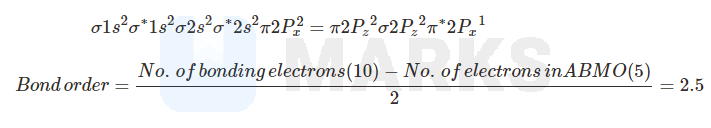Search any question & find its solution
Bond order of the covalent molecule, is given as , As is a covalent diatomic molecule of second row elements of periodic table we can consider but bond order of having electrons which is isoelectronic to nitrogen should be equal to and having 15 electrons will have a bond order of .
Molecular orbital configuration and bond order calculation of is
ABMO antibonding molecular orbital
Bond order Number of bonding electrons number of anti bonding electrons.
Note: Total number of electrons equal to 13 will also have the 2.5 bond order. But in this case neutral diatomic molecule will not be possible
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.