Search any question & find its solution
Question:
Answered & Verified by Expert
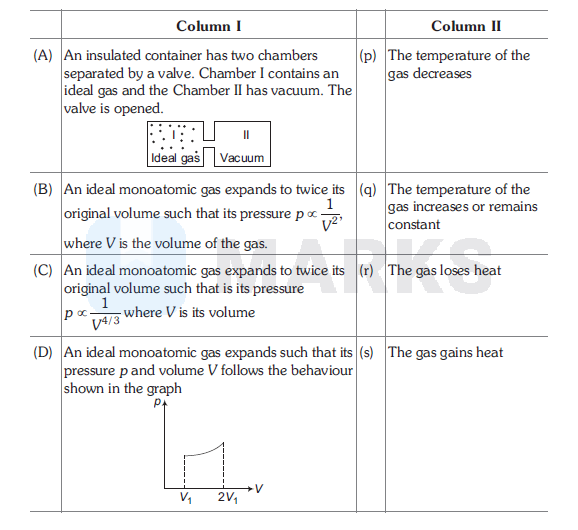
Column I contains a list of processes involving expansion of an ideal gas. Match this with Column II describing the thermodynamic change during this process. Indicate your answer by darkening the appropriate bubbles of the $4 \times 4$ matrix given in the ORS.
Options:

Solution:
2092 Upvotes
Verified Answer
The correct answer is:
(A) q, (B) p,q, (C) p,s, (D) q,s
(A) q, (B) p,q, (C) p,s, (D) q,s
(A) : In case of free expansion under adiabatic conditions, change in internal energy $\Delta U=0$.
$\therefore$ Internal energy and temperature will remain constant.
(B) $p \propto \frac{1}{v^2}$
$$
\begin{array}{rrrr}
& \therefore & p V^2 & =\text { constant } \\
& \text { or } & \left(\frac{n R T}{V}\right) V^2=\text { constant } \\
& \therefore & T \propto \frac{1}{V}
\end{array}
$$
If volume is doubled, temperature will decrease as per Eq. (ii). Further, molar heat capacity in process $p V^x=$ constant is
$$
C=C_V+\frac{R}{1-x}
$$
From Eq. (i), $x=2$
$$
\therefore \quad C=\frac{3}{2} R+\frac{R}{1-2}=+\frac{R}{2}
$$
Since molar heat capacity is positive, according to $Q=n C \Delta T, Q$ will be negative it $\Delta T$ is negative. Or gas loses heat if temperature is decreasing.
(C) :
$$
\begin{aligned}
p & \propto \frac{1}{V^{1 / 3}} \\
P V^{4 / 3} & =\text { constant } \\
\left(\frac{n R T}{V}\right) V^{4 / 3} & =\text { constant } \\
T & \propto \frac{1}{V^{1 / 3}}
\end{aligned}
$$
Further, with increase in volume temperature will decrease.
Here,
$$
\begin{array}{lc}
\text { Here, } & x=4 / 3 \\
\therefore & C=\frac{3}{2} R+\frac{R}{1-4 / 3}=-1.5 R
\end{array}
$$
As molar heat capacity is negative, $Q$ will be positive, if $\Delta T$ is negative or gas gains heat with decrease in temperature.
(D) $T \propto p V$
In expansion from $V_1$ or $2 V_1$, product of $p V$ is increasing. Therefore, temperature will increase or $\Delta U=+$ ve.
Further, in expansion work done is also positive.
Hence, $Q=W+\Delta U=+$ ve
or gas gains heat.
$\therefore$ Internal energy and temperature will remain constant.
(B) $p \propto \frac{1}{v^2}$
$$
\begin{array}{rrrr}
& \therefore & p V^2 & =\text { constant } \\
& \text { or } & \left(\frac{n R T}{V}\right) V^2=\text { constant } \\
& \therefore & T \propto \frac{1}{V}
\end{array}
$$
If volume is doubled, temperature will decrease as per Eq. (ii). Further, molar heat capacity in process $p V^x=$ constant is
$$
C=C_V+\frac{R}{1-x}
$$
From Eq. (i), $x=2$
$$
\therefore \quad C=\frac{3}{2} R+\frac{R}{1-2}=+\frac{R}{2}
$$
Since molar heat capacity is positive, according to $Q=n C \Delta T, Q$ will be negative it $\Delta T$ is negative. Or gas loses heat if temperature is decreasing.
(C) :
$$
\begin{aligned}
p & \propto \frac{1}{V^{1 / 3}} \\
P V^{4 / 3} & =\text { constant } \\
\left(\frac{n R T}{V}\right) V^{4 / 3} & =\text { constant } \\
T & \propto \frac{1}{V^{1 / 3}}
\end{aligned}
$$
Further, with increase in volume temperature will decrease.
Here,
$$
\begin{array}{lc}
\text { Here, } & x=4 / 3 \\
\therefore & C=\frac{3}{2} R+\frac{R}{1-4 / 3}=-1.5 R
\end{array}
$$
As molar heat capacity is negative, $Q$ will be positive, if $\Delta T$ is negative or gas gains heat with decrease in temperature.
(D) $T \propto p V$
In expansion from $V_1$ or $2 V_1$, product of $p V$ is increasing. Therefore, temperature will increase or $\Delta U=+$ ve.
Further, in expansion work done is also positive.
Hence, $Q=W+\Delta U=+$ ve
or gas gains heat.
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.