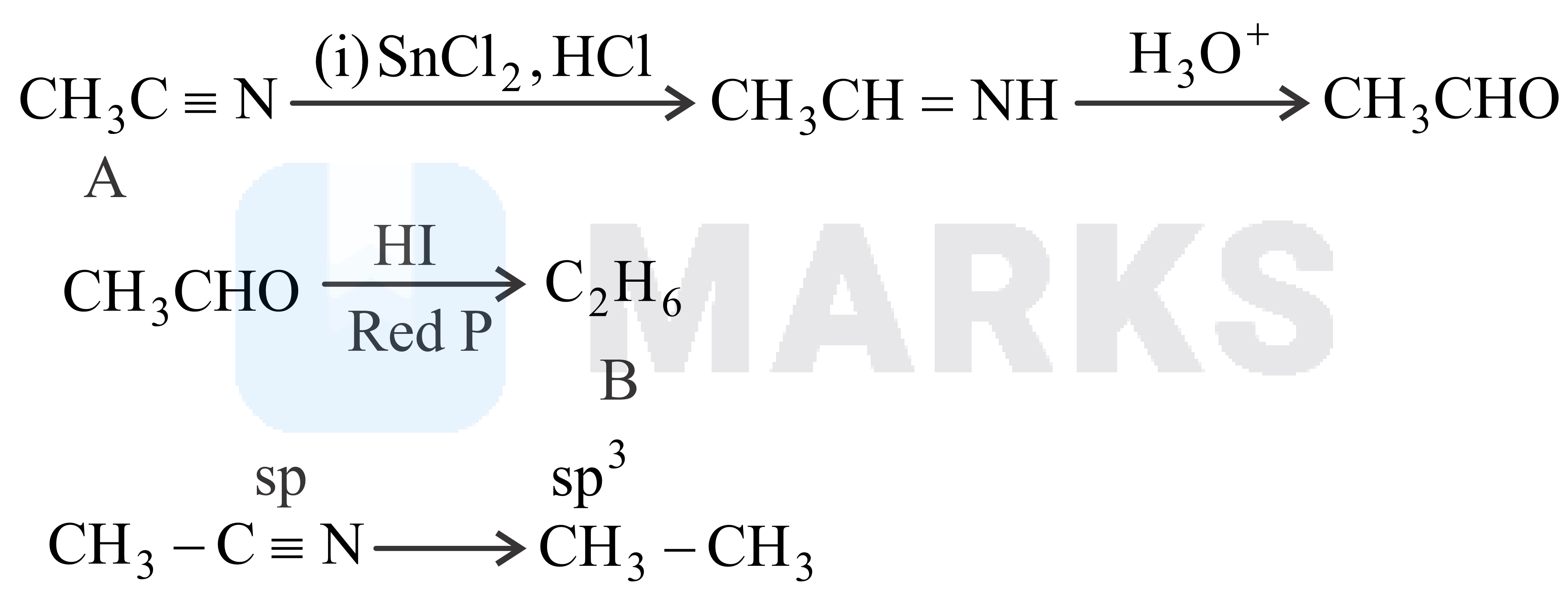Search any question & find its solution
Consider following reaction, where
(A) The change in the functional group and
(B) The corresponding change in the hybridization from starting to the final product and are
Nitriles are reduced to corresponding imine with stannous chloride in the presence of hydrochloric acid, which on hydrolysis give corresponding aldehyde.
This reaction is called Stephen reaction.
When ethane nitrile undegoes stephen reduction ethanal is produced.
Ethanal on reaction with HI in presence of red phosphorous produces ethane.
The hybridization of carbon in nitrile group is sp and in ethane sp3.
The change in functional group is from -CN to -CH3
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.