Search any question & find its solution
Question:
Answered & Verified by Expert
Consider the bcc unit cells of the solids and with the position of atoms as shown below. The radius of atom is twice that of atom The unit cell edge length is more in solid than in . What is the approximate packing efficiency in solid ?
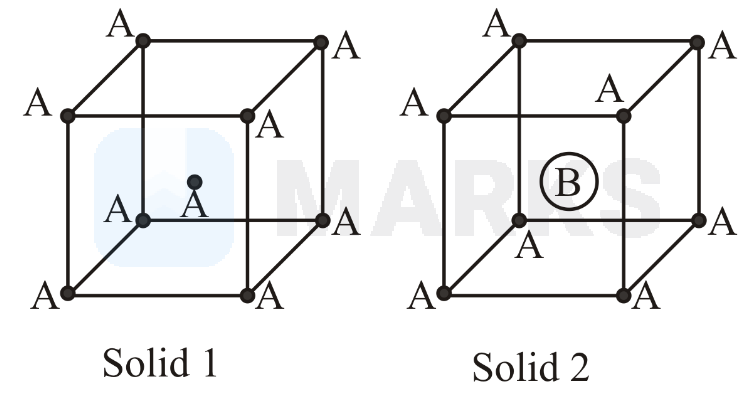
Solution:
2500 Upvotes
Verified Answer
The correct answer is:
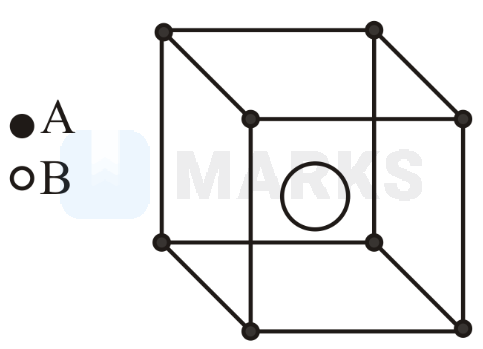
If radius of is radius of will be . Atoms in the body diagonal touch.
Therefore:
or
Packing Efficiency
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.