Search any question & find its solution
Question:
Answered & Verified by Expert
Consider the following bromides :
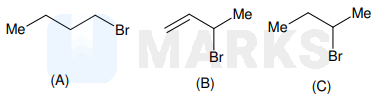
The correct order of $\mathrm{S}_{\mathrm{N}} 1$ reactivity is
Options:

The correct order of $\mathrm{S}_{\mathrm{N}} 1$ reactivity is
Solution:
1401 Upvotes
Verified Answer
The correct answer is:
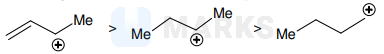
$B>C>A$
$B>C>A$
$\mathrm{S}_{\mathrm{N}} 1$ proceeds via carbocation intermediate, the most stable one forming the product faster. Hence reactivity order for $\mathrm{A}, \mathrm{B}, \mathrm{C}$ depends on stability of carbocation created.

Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.