Search any question & find its solution
Question:
Answered & Verified by Expert
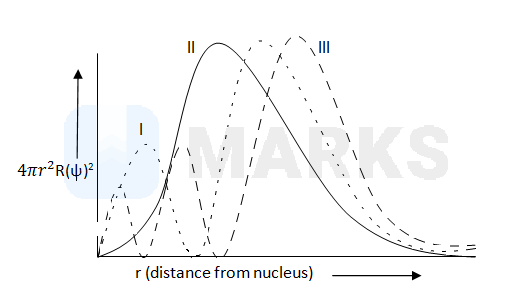
Consider the following radial distribution function diagrams. Which of the following has the correct matching of curve and orbital ?
Options:

Solution:
2294 Upvotes
Verified Answer
The correct answer is:
I(3p), II(3d), III(3s)
No of radial node = n - ℓ - 1 .
I(3p) ---- n = 3 and ℓ =1. One radial node
II(3d) ---- n = 3 and ℓ =2. No radial node
III(3s) ---- n = 3 and ℓ =0. Two radial nodes.
All the orbitals provided in choices belong to match. I(3p), II(3d), III(3s)
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.