Search any question & find its solution
Question:
Answered & Verified by Expert
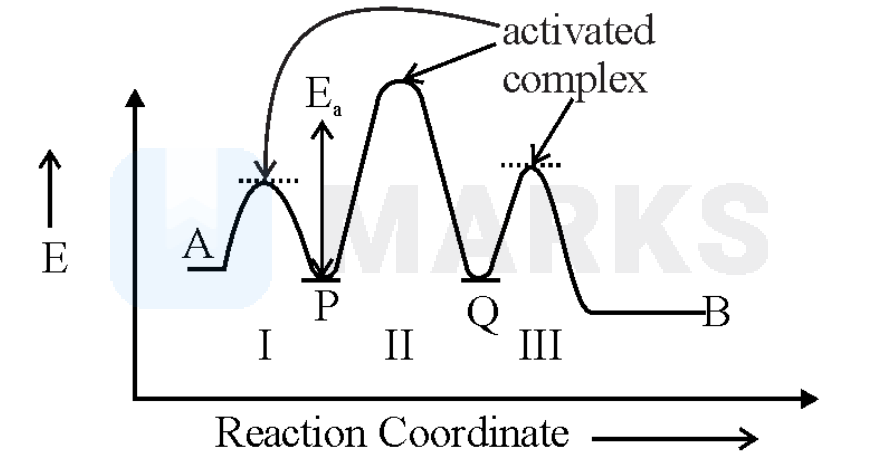
Consider the following reaction that goes from to in three steps as shown below:
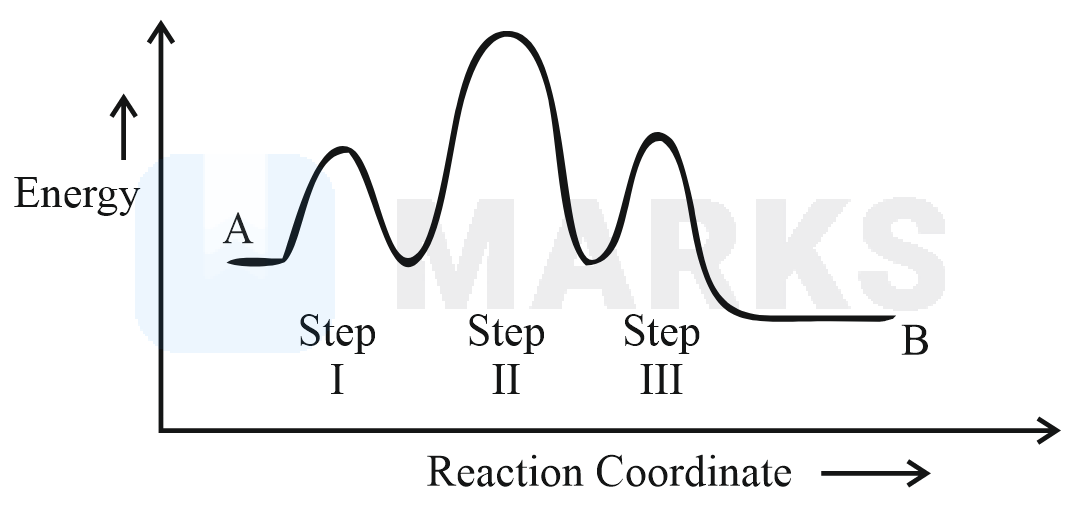
Choose the correct option
| Number of Intermediates | Number of Activated Complexes | Rate determining step | |
Solution:
1785 Upvotes
Verified Answer
The correct answer is:
The curve contain three activated complex as shown below in the curve.In the curve P and Q are the intermediates. The step with the highest activation energy is the rate determining step. Hence, in the given reaction, two intermediates, three activated complexes are involved and step-II is the rate determining step.
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.