Search any question & find its solution
Question:
Answered & Verified by Expert
Consider the following reactions:
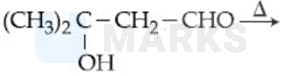
Which of the reaction(s) will not produce Saytzeff product?
Solution:
2962 Upvotes
Verified Answer
The correct answer is:
only
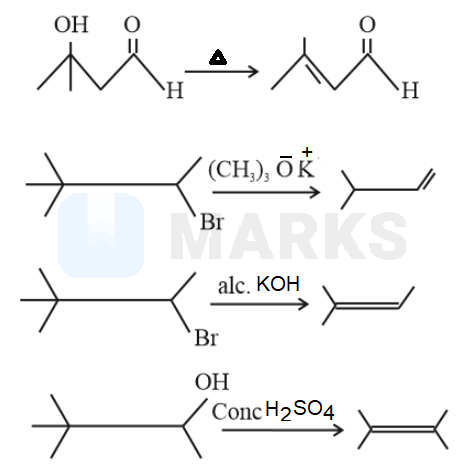
We have to know that a Saytzeff product is basically an alkene compound which has undergone many substitution reactions to form the alkene product. From Saytzeff rule, a stable alkene is formed when removal of hydrogen from beta carbon has a low number of hydrogen atoms.
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.