Search any question & find its solution
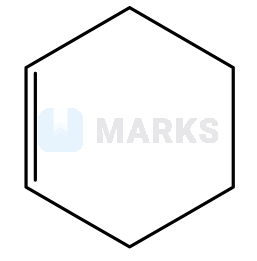 is _________ type of an organic compound.
is _________ type of an organic compound.Benzenoid aromatic compounds: The benzenoid aromatic compounds are referred to as those compounds which contain only benzene rings in the whole structure. The benzene ring may be one or more than one. Examples of Benzenoid aromatic compounds are benzene-benzene contains a single ring, naphthalene- naphthalene contains two benzene rings.
Non-benzenoid aromatic compounds: The non-benzenoid aromatic compounds are referred to as those compounds which do not contain any benzene ring in their structure. Example Furan- Furan is a five membered ring which contains four carbon atoms and one oxygen atom.
For a compound to be aromatic it should follow Huckel’s rule. The Huckel rule says that for a ring to be aromatic in nature it should follow the given rules shown below.
(1) The ring should be planar
(2) The ring should be complete delocalized by pi-electrons
(3) The compound should possess
electrons in the ring.
Acyclic compounds are just opposite to cyclic compounds because their molecules don't form any ring. It is called open-chain compounds because they have a linear structure.
Alicyclic compounds are organic compounds that are both aliphatic and cyclic. These are the saturated or unsaturated,hydrocarbons containing non-aromatic rings of carbon atoms. Alicyclic compounds may have one or more aliphatic side chains attached.
 is Alicyclic
is Alicyclic
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.