Search any question & find its solution
Question:
Answered & Verified by Expert
.
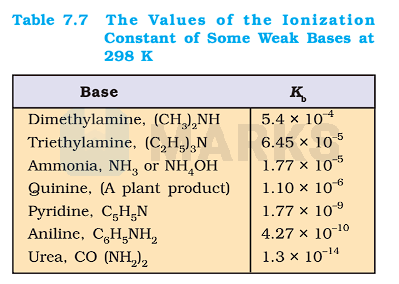
Determine the degree of ionization and pH of a 0.05M of ammonia solution. The ionization constant of ammonia can be taken from Table 7.7.
Options:

Determine the degree of ionization and pH of a 0.05M of ammonia solution. The ionization constant of ammonia can be taken from Table 7.7.
Solution:
1306 Upvotes
Verified Answer
The correct answer is:
Degree of ionization 0.018%, pH = 11
Correct Option is : (A)
Degree of ionization 0.018%, pH = 11
Degree of ionization 0.018%, pH = 11
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.