Search any question & find its solution
Question:
Answered & Verified by Expert
Dihydrogen gas used in Haber's process is produced by reacting methane from natural gas with high temperature steam. The first stage of two stage reaction involves the formation of \(\mathrm{CO}\) and \(\mathrm{H}_2\). In second stage, \(\mathrm{CO}\) formed in first stage is reacted with more steam in water gas shift reaction.
\(\mathrm{CO}(\mathrm{g})+\mathrm{H}_2 \mathrm{O}(\mathrm{g}) \rightleftharpoons \mathrm{CO}_2(\mathrm{~g})+\mathrm{H}_2(\mathrm{~g})\).
If a reaction vessel at \(400^{\circ} \mathrm{C}\) is charged with an equimolar mixture of \(\mathrm{CO}\) and steam such that \(\mathrm{p}_{\mathrm{CO}}=\mathrm{P}_{\mathrm{H}_2 \mathrm{O}}=\mathbf{4 . 0}\) bar, what will be the partial pressure of \(\mathrm{H}_2\) at equilibrium ?
\(K_p=10.1\) at \(400^{\circ} \mathrm{C}\).
\(\mathrm{CO}(\mathrm{g})+\mathrm{H}_2 \mathrm{O}(\mathrm{g}) \rightleftharpoons \mathrm{CO}_2(\mathrm{~g})+\mathrm{H}_2(\mathrm{~g})\).
If a reaction vessel at \(400^{\circ} \mathrm{C}\) is charged with an equimolar mixture of \(\mathrm{CO}\) and steam such that \(\mathrm{p}_{\mathrm{CO}}=\mathrm{P}_{\mathrm{H}_2 \mathrm{O}}=\mathbf{4 . 0}\) bar, what will be the partial pressure of \(\mathrm{H}_2\) at equilibrium ?
\(K_p=10.1\) at \(400^{\circ} \mathrm{C}\).
Solution:
1067 Upvotes
Verified Answer
Suppose the partial pressure of \(\mathrm{H}_2\) at equilibrium \(=\mathrm{p}\) bar
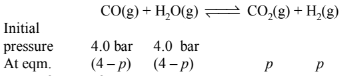
\(\mathrm{K}_{\mathrm{p}}=p^2 /(4-p)^2=10.1\) (Given)
\(\therefore \mathrm{p} /(4-p)=\sqrt{10.1}=3.178\)
or \(p=3.042 \mathrm{bar}\)
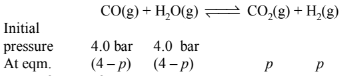
\(\mathrm{K}_{\mathrm{p}}=p^2 /(4-p)^2=10.1\) (Given)
\(\therefore \mathrm{p} /(4-p)=\sqrt{10.1}=3.178\)
or \(p=3.042 \mathrm{bar}\)
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.