Search any question & find its solution
Question:
Answered & Verified by Expert
Energy of activation of forward reaction for an endothermic process is $50 \mathrm{~kJ}$. If enthalpy change for forward reaction is $20 \mathrm{~kJ}$ then enthalpy change for backward reaction will be
Options:
Solution:
2683 Upvotes
Verified Answer
The correct answer is:
$30 \mathrm{~kJ}$
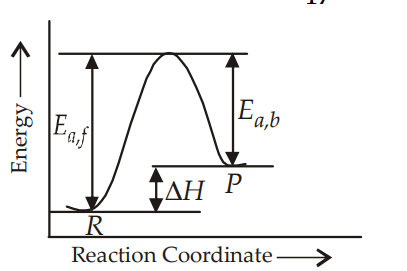
For endothermic reaction,
$E_{a, b}=E_{a, f}-\Delta H^{\circ}$
$=50 \mathrm{~kJ}-20 \mathrm{~kJ}=30 \mathrm{~kJ}$
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.