Search any question & find its solution
Question:
Answered & Verified by Expert
Explain why propanol has higher boiling point than that of the hydrocarbon, butane?
Solution:
1532 Upvotes
Verified Answer
The molecules of butane are held together by weak van der Waal's forces of attraction while those of propanol are held together by stronger intermolecular hydrogen bonding.
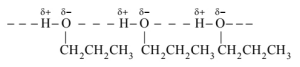
Therefore, the boiling point of propanol is much higher than that of butane.
Therefore, the boiling point of propanol is much higher than that of butane.
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.