Search any question & find its solution
Question:
Answered & Verified by Expert
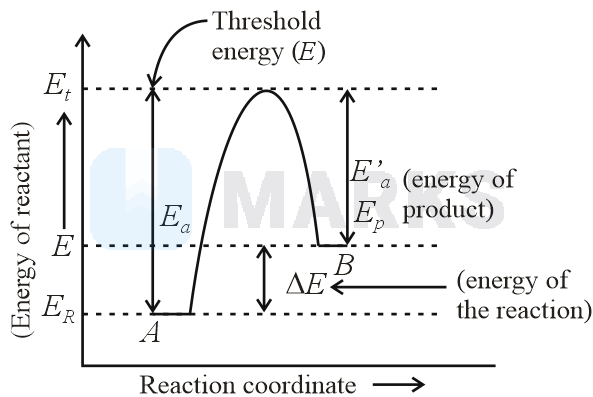
For a reversible reaction, , which one of the following statement is wrong from the given energy profile diagram?
Solution:
1390 Upvotes
Verified Answer
The correct answer is:
The threshold energy is less than that of activation energy

where, activation energy of forward reaction
activation energy of backward reaction
The above energy profile diagram shows that
The potential energy of the product is greater than that of the reactant, so the reaction is endothermic.
or
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.