Search any question & find its solution
Question:
Answered & Verified by Expert
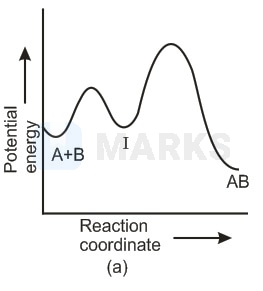
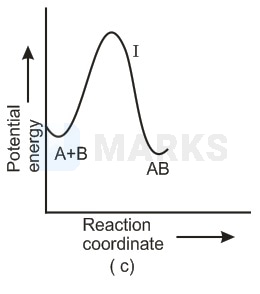
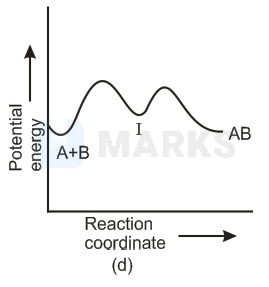
For an exothermic reaction, following two steps are involved.
Which of the following graphs correctly represent this reaction ?
Options:
Which of the following graphs correctly represent this reaction ?
Solution:
1240 Upvotes
Verified Answer
The correct answer is:


In any reaction mechanism the no. of activated complexes showing maxima of potential energies is the no. of steps in that mechanism and in between these maxima is a valley where lies a more stable intermediate. There is one reaction intermediate lying at valley of two maxima representing two steps. Ea for first step should be higher than second step as first is slow step. These requirements fulfilled by choice (ii) & not choice (i) the choice (iv) is not possible because that is for endothermic reaction & (ii) is single step equation so not possible.
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.