Search any question & find its solution
Question:
Answered & Verified by Expert
For three low density gases pressure versus temperature graphs are plotted while keeping them at constant volume, as shown in the figure
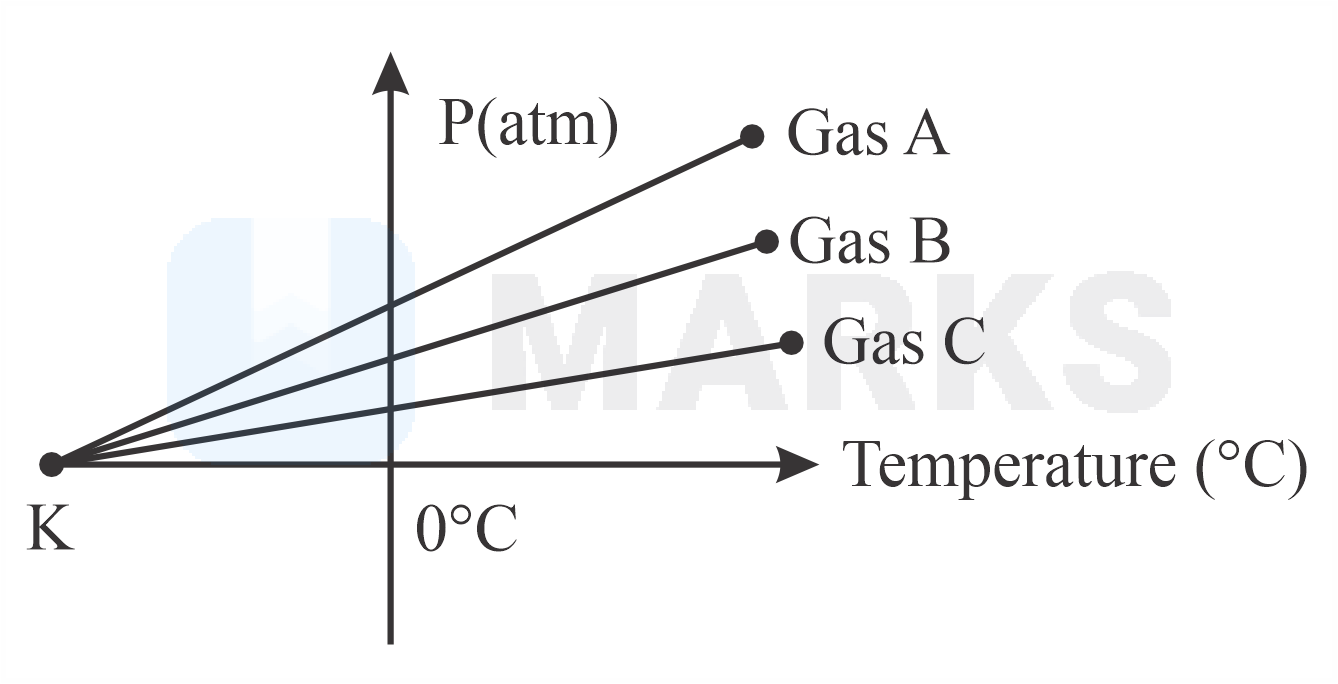
The temperature corresponding to the point is:
Solution:
1189 Upvotes
Verified Answer
The correct answer is:
In case of constant volume process, i.e., isochoric process,
From ideal gas equation,
constant
Or we have,
If
Thus, when temperature goes to , all constituent particles get freezed and pressure goes to zero irrespective of the density of gas.
Hence, the temperature corresponding to the point .
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.