Search any question & find its solution
Given below are two statements :
Statement (I) : Dimethyl glyoxime forms a six membered covalent chelate when treated with solution in presence of
Statement (II) : Prussian blue precipitate contains iron both in and oxidation states.
In the light of the above statements, choose the most appropriate answer from the options given below:
According to Statement I,
On addition of dimethylglyoxime (DMG) to nickel ion solution and on adding little bit of ammonia to make solution basic, it will give a red precipitate. This is a very specific test for nickel cation.
The reaction is as follows,
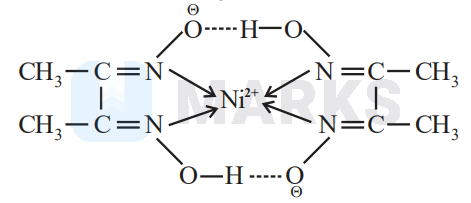
hydrogen bonded chelatesare formed.
According to Statement II,
Prussian Blue
IN this complex Fe is at oxidation state. Where ionisation sphere is and coordination sphere has
Hence option A is the answer..
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.