Search any question & find its solution
Question:
Answered & Verified by Expert
How many statements are true for the following pair of compounds?
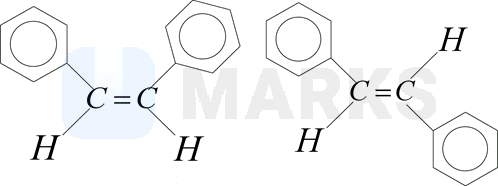
Cis Trans
(i) The dipole moment of trans isomer is zero
(ii) The boiling point of cis isomer is more than trans isomer
(iii) Cis isomer is more stable than the trans isomer
(iv) These are also called configurational diastereomers
(v) These are readily interconvertible under normal conditions
(vi) The melting point of trans isomer is more than the cis isomer
(vii) Trans isomer is more soluble than cis isomer in polar solvents

Cis Trans
(i) The dipole moment of trans isomer is zero
(ii) The boiling point of cis isomer is more than trans isomer
(iii) Cis isomer is more stable than the trans isomer
(iv) These are also called configurational diastereomers
(v) These are readily interconvertible under normal conditions
(vi) The melting point of trans isomer is more than the cis isomer
(vii) Trans isomer is more soluble than cis isomer in polar solvents
Solution:
2175 Upvotes
Verified Answer
The correct answer is:
4
(i) The dipole moment of trans isomer is zero
(ii) The boiling point of cis isomer is more than trans isomer
(iii) These are also called configurational diastereomers
(vi) The melting point of trans isomer is more than the cis isomer
these are the true statements.
(ii) The boiling point of cis isomer is more than trans isomer
(iii) These are also called configurational diastereomers
(vi) The melting point of trans isomer is more than the cis isomer
these are the true statements.
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.