Search any question & find its solution
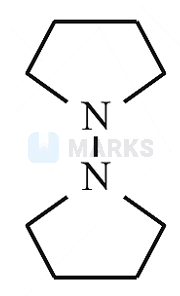
Identify B formed in the reaction.
Substituted ammonium salt is formed when chloroalkane reacted with excess of , which on reaction with gives .
The carbon - halogen bond in alkyl halides can be easily cleaved by a nucleophile. Hence, an alkyl halide on reaction with an ethanolic solution of ammonia undergoes a nucleophilic substitution reaction in which the halogen atom is replaced by an amino group. This process of cleavage of the bond by ammonia molecule is known as ammonolysis. Substituted ammonium salt on reaction with sodium hydroxide gives 1,4-diaminobutane as shown below,
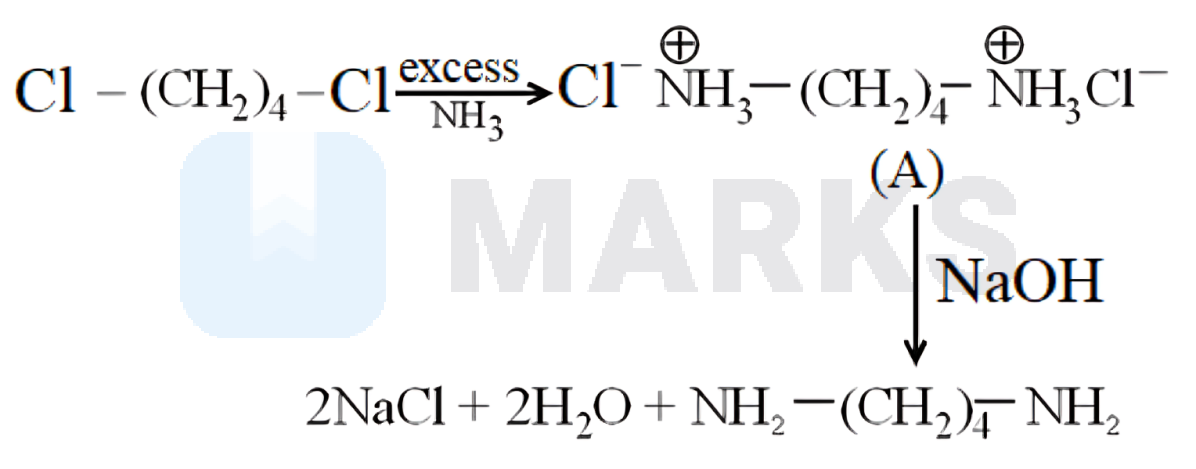
Hence, the answer is option B.
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.