Search any question & find its solution
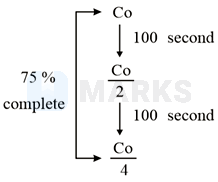
50% of a reaction occurs in 100 second and 75% of the reaction occurs in 200 second, the order of this reaction is 1.
Let the initial reactant concentration is 1 M.
In the first 100 seconds, reactant concentration is reduced to
In the second 100 seconds (total 200 seconds from start), reactant concentration is reduced to .
Thus, we observe that after each 100 second period, the reactant concentration is reduced to one half. Hence, 100 seconds is the half-life period and is independent of reactant concentration. This is characteristic of the first-order reaction.
First-order reaction as the half-life is constant.
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.