Search any question & find its solution
Question:
Answered & Verified by Expert
If an iron (III) complex with the formula \(\left[\mathrm{Fe}\left(\mathrm{NH}_3\right)_x(\mathrm{CN})_y\right]^{-}\)has no electron in its \(e_g\) orbital, then the value of \(x+y\) is
Options:
Solution:
1508 Upvotes
Verified Answer
The correct answer is:
6
Complex is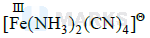
$\begin{aligned} & x=2 \\ & y=4 \\ & \text { so } x+y=6\end{aligned}$
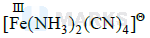
$\begin{aligned} & x=2 \\ & y=4 \\ & \text { so } x+y=6\end{aligned}$
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.