Search any question & find its solution
Question:
Answered & Verified by Expert
If enthalpy of atomization for is and bond enthalpy for is , the relation between them
Options:
Solution:
2080 Upvotes
Verified Answer
The correct answer is:
is .
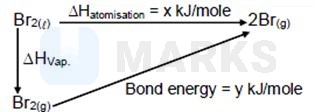
Hence
Enthalpy of atomization is enthalpy change involved when one mole of gaseous atom is formed from a substance in an elemental state (Elemental state of Bromine is ).
While, bond energy is energy required to break one mole of bonds in gaseous state to form separated atoms.
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.