Search any question & find its solution
Question:
Answered & Verified by Expert
If one mole of ammonia and one mole of hydrogen chloride are mixed in a closed container to form ammonium chloride gas, then
Options:
Solution:
2821 Upvotes
Verified Answer
The correct answer is:
$\Delta H < \Delta U$
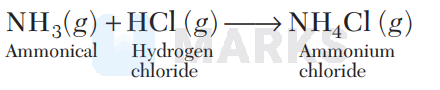
$\quad \Delta n_{g}=n_{P}-n_{R}=1-2=-1$
Also, $\Delta H=\Delta U+\Delta n_{g} R T$
$\therefore \quad \Delta H-\Delta U=-\mathrm{Re} \quad\left[\quad \Delta n_{g}=-\mathrm{ve}\right]$
$\therefore \quad \Delta M < \Delta U$
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.