Search any question & find its solution
Question:
Answered & Verified by Expert
If the dipole moment of Toluene and Nitro-benzene are 0.43 D and 3.93 D respectively, then what is the expected dipole moment of p-Nitrotoluene?
Options:
Solution:
1825 Upvotes
Verified Answer
The correct answer is:
4.36 D
Methyl group has effect and group has effect Therefore, in p-nitro toluene the dipole moments of and groups act in the same direction. So, the resultant dipole moment is additive.
i.e., 3.93 + 0.43 = 4.36 debye
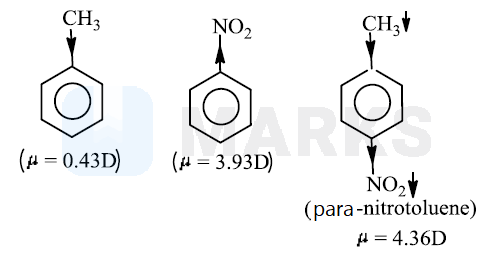
i.e., 3.93 + 0.43 = 4.36 debye

Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.