Search any question & find its solution
Question:
Answered & Verified by Expert
If Z is the compressibility factor, then Van der Waal's equation at low pressure can be written as:
Options:
Solution:
2784 Upvotes
Verified Answer
The correct answer is:
Van der Waal's Equation of state for one mole
At low pressure, the characteristic constant (a) becomes significant whereas (b) becomes negligible. So (b) is ignored.
At low pressure for most gases z < 1.
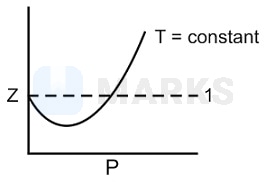
At low pressure, the characteristic constant (a) becomes significant whereas (b) becomes negligible. So (b) is ignored.
At low pressure for most gases z < 1.

Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.