Search any question & find its solution
Question:
Answered & Verified by Expert
In a hypothetical solid, C atoms are found to form cubical close-packed lattice. A atoms occupy all tetrahedral voids and B atoms occupy all octahedral voids.
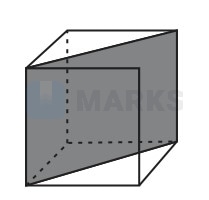
A and B atoms are of appropriate size, so that there is no distortion in the CCP lattice of C atoms. Now, if a plane as shown in the following figure is cut, then the cross section of this plane will look like
Options:

A and B atoms are of appropriate size, so that there is no distortion in the CCP lattice of C atoms. Now, if a plane as shown in the following figure is cut, then the cross section of this plane will look like
Solution:
2640 Upvotes
Verified Answer
The correct answer is:
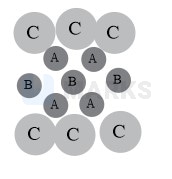

From figure, it is clear that 4 corners and 2 face centers lie on the shaded plane. Therefore, there will be six C atoms, and atoms (marked A) in TVs do not touch other.
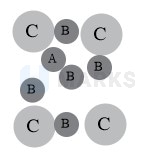
Fig (i) is not possible; four atom marked C.
Fig. (ii) is not possible, atoms A in TVs are not shown in figure.
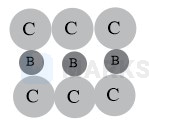
Fig. (iii) is possible, since atoms A in TVs are not touching each other. There are four atoms A on two body diagonals contained in shades plane.
Fig (iv) is not possible, since atoms A in TVs are touching each other.
Fig (i) is not possible; four atom marked C.
Fig. (ii) is not possible, atoms A in TVs are not shown in figure.
Fig. (iii) is possible, since atoms A in TVs are not touching each other. There are four atoms A on two body diagonals contained in shades plane.
Fig (iv) is not possible, since atoms A in TVs are touching each other.
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.