Search any question & find its solution
Question:
Answered & Verified by Expert
In a thermally isolated system, two boxes filled with an ideal gas are connected by a valve. When the valve is in closed position, states of the box 1 and 2 , respectively, are (1 atm, V, T) and (0.5 atm, $4 \mathrm{~V}, \mathrm{~T}$ ). When the valve is opened, the final pressure of the system is approximately
Options:
Solution:
2421 Upvotes
Verified Answer
The correct answer is:
$0.6 \mathrm{~atm}$
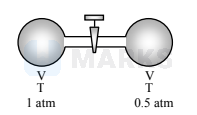
After opening of at equilibrium temperature and pressure of whole gas is $\mathrm{T}_{1}$ and $\mathrm{P}_{1}$
$\begin{array}{l}
n_{1}=\frac{1 \times V}{R T}, n_{2}=\frac{0.5 \times V \times 4}{R T} \\
n_{1}+n_{2}=n \\
\frac{V}{R T}+\frac{V \times 4}{2 R T}=\frac{5 V P_{1}}{R T_{1}} \\
\frac{3 V}{R T}=\frac{5 V P_{1}}{R T_{1}} \\
\frac{P_{1}}{T_{1}}=\frac{0.6}{T} \\
\Delta Q=0, \quad \Delta W=0 \\
\therefore \Delta U=0 \\
n_{1} C_{V} T+n_{2} C_{V} T=\left(n_{1}+n_{2}\right) C_{V} T_{1} \\
T_{1}=T \\
\frac{P_{1}}{T}=\frac{0.6}{T} \\
P_{1}=0.6 \text { atm }
\end{array}$
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.