Search any question & find its solution
Question:
Answered & Verified by Expert
In chromyl chloride test for confirmation of ion, a yellow solution is obtained. Acidification of the solution and addition of amyl alcohol and turns organic layer blue indicating formation of chromium pentoxide. The oxidation state of chromium in that is
Options:
Solution:
1727 Upvotes
Verified Answer
The correct answer is:
The chromyl chloride test is used to detect chloride ions in the qualitative analysis. If any chloride salt like sodium chloride is heated with acidified potassium dichromate it produces red colour fumes of chromyl chloride. It confirms the presence of chloride ions in that salt.
Chromate reacts rapidly with hydrogen peroxide to exchange two oxo ligands and become diperoxochromate(VI)
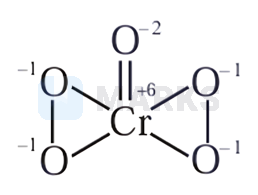
Hence, the answer is option A.
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.