Search any question & find its solution
Question:
Answered & Verified by Expert
In $\left[\mathrm{CO}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}$, there are three unpaired electrons present. The $\mu_{\text {calculated }}$ is 3.87 BM which is quite different from the $\mu_{\text {experimental }}$ of 4.40 BM .
This is because of
Options:
This is because of
Solution:
1021 Upvotes
Verified Answer
The correct answer is:
some contribution of the orbital motion of the electron to the magnetic moment
In $\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}$, there are three unpaired electrons present as,
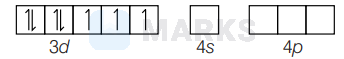
$\mu_{\mathrm{cal}}=\sqrt{n(n+1)} \mathrm{BM}=\sqrt{3(3+1)} \mathrm{BM}=3.87 \mathrm{BM}$
As, the $t_{2 g}$ orbital is unsymmetrically filled hence due to orbital motion contribution, magnetic moment goes with the value 4.40 BM
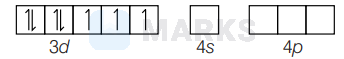
$\mu_{\mathrm{cal}}=\sqrt{n(n+1)} \mathrm{BM}=\sqrt{3(3+1)} \mathrm{BM}=3.87 \mathrm{BM}$
As, the $t_{2 g}$ orbital is unsymmetrically filled hence due to orbital motion contribution, magnetic moment goes with the value 4.40 BM
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.