Search any question & find its solution
Question:
Answered & Verified by Expert
In $\mathrm{PO}_{4}^{3-},$ the formal charge on each oxygen atom and the $\mathrm{P}-\mathrm{O}$ bond order respectively are
Options:
Solution:
2300 Upvotes
Verified Answer
The correct answer is:
-0.75,1.25
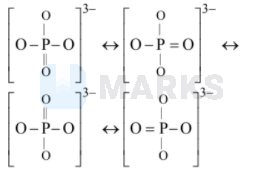
Bond order
$=\frac{\text { Number of bonds }}{\text { Number of Resonating structures }}$
$=\frac{5}{4}=1.25$
Three unit negative charge is being shared by four $\mathrm{O}$ atoms. Formal charge $=-3 / 4$
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.