Search any question & find its solution
Question:
Answered & Verified by Expert
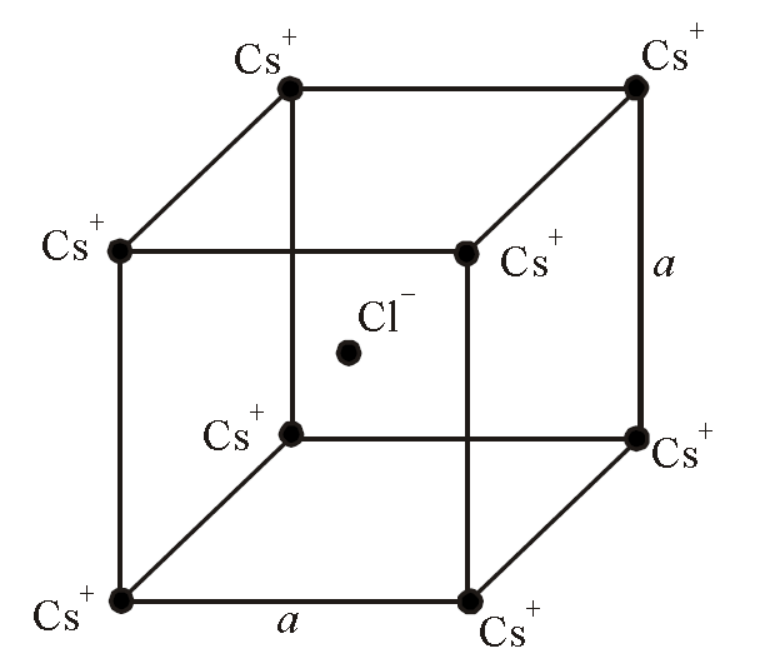
In the basic $\mathrm{CsCl}$ crystal structure, $\mathrm{Cs}^{+}$ and $\mathrm{Cl}^{-}$ions are arranged in a bcc configuration as shown in the figure. The net electrostatic force exerted by the eight $\mathrm{Cs}^{+}$ions on the $\mathrm{Cl}^{-}$ions is
Options:

Solution:
2896 Upvotes
Verified Answer
The correct answer is:
zero.
The electrostatic force due to one $\mathrm{Cs}^{+}$ion is balanced by diagonally opposite other $\mathrm{Cs}^{+}$. Thus the net electrostatic force on $\mathrm{Cl}^{-}$ion due to eight $\mathrm{Cs}^{+}$ ions is zero.
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.