Search any question & find its solution
Question:
Answered & Verified by Expert
In the face centered unit cell, the lattice points are present at
Options:
Solution:
1249 Upvotes
Verified Answer
The correct answer is:
the corners and the face centres of the unit cell
Simplest crystal is to be studied in cubic system. Three types of cubic systems are following:-
Simple Cubic : Atoms are arranged at the corners of the cube.
Body Centered Cubic : Atoms are arranged at the corners and at the centre of the cube.
Face Centered Cubic : Atoms are arranged at the corners and at centered of the each faces.
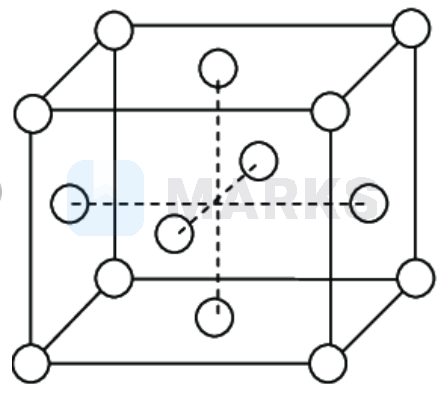
Hence, in the face centered unit cell, the lattice points are present at the corners and the face centres of the unit cell.
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.