Search any question & find its solution
Question:
Answered & Verified by Expert
In the following compounds
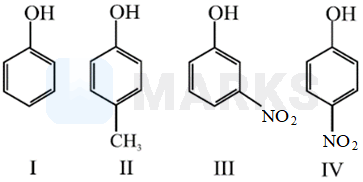
The order of acidity is
Options:

The order of acidity is
Solution:
2417 Upvotes
Verified Answer
The correct answer is:
IV > III > I > II
is an electron attracting group whereas is an electron releasing group. An electron attracting substituent tends to disperse the negative charge of phenoxide ion and thus makes it more stable. This in turn increases the acidity of phenol. The substituent in Para-position is more effective than in meta-position as the former involves a resonating structure bearing negative charge on the carbon attached to electron with drawing substituent.
An electron releasing substituent tends to increase the negative charge of peroxide ion and thus makes it more unstable. This, in turn, decreases the acid strength of phenol.
An electron releasing substituent tends to increase the negative charge of peroxide ion and thus makes it more unstable. This, in turn, decreases the acid strength of phenol.
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.