Search any question & find its solution
Question:
Answered & Verified by Expert
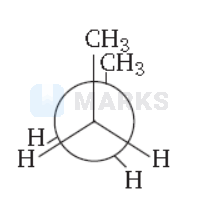
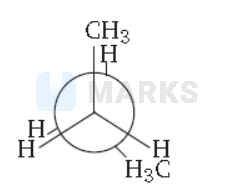
In the following the most stable conformation of n-butane is
Options:
Solution:
1068 Upvotes
Verified Answer
The correct answer is:
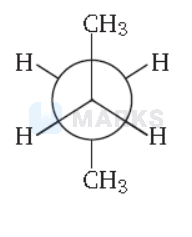

Key Idea The conformation in which the heavier groups are present at maximum possible distances, so that the forces of repulsion get weak, is more stable.
Among the given conformations of n-butane, the conformation shown in option (b) ie, anti conformation is most stable as in it the bulkier group (ie, $\mathrm{CH}_3$ group) are present at maximum possible distance.
Among the given conformations of n-butane, the conformation shown in option (b) ie, anti conformation is most stable as in it the bulkier group (ie, $\mathrm{CH}_3$ group) are present at maximum possible distance.
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.