Search any question & find its solution
Question:
Answered & Verified by Expert
In the Kolbe electrolysis of sodium propanoate, the products $\mathrm{X}$ and $\mathrm{Y}$ are formed at respected electrodes. What are $\mathrm{X}$ and $\mathrm{Y}$ ?
Options:
Solution:
1919 Upvotes
Verified Answer
The correct answer is:
$\mathrm{X}=\mathrm{CH}_3-\mathrm{CH}_2-\mathrm{CH}_2-\mathrm{CH}_3$ at Anode; $\mathrm{Y}=\mathrm{H}_2$ at Cathode
$2 \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CO} \overline{\mathrm{O}^2} \mathrm{Na} \xrightarrow[2 \mathrm{H}_2 \mathrm{O}]{\text { electrolysis }}$
$$
\mathrm{CH}_3 \mathrm{CH}_2-\mathrm{CH}_2 \mathrm{CH}_3+2 \mathrm{CO}_2+\mathrm{H}_2+2 \mathrm{NaOH}
$$
At anode (oxidation):
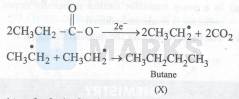
At cathode (reduction):
$$
\begin{aligned}
& 2 \mathrm{H}_2 \mathrm{O}+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{OH}^{-}+2 \mathrm{H}^{+} \\
& 2 \mathrm{H}^{+} \rightarrow \mathrm{H}_2(\mathrm{Y}) \\
& 2 \mathrm{Na}^{+}+2 \mathrm{OH}^{-} \rightarrow 2 \mathrm{NaOH}
\end{aligned}
$$
$$
\mathrm{CH}_3 \mathrm{CH}_2-\mathrm{CH}_2 \mathrm{CH}_3+2 \mathrm{CO}_2+\mathrm{H}_2+2 \mathrm{NaOH}
$$
At anode (oxidation):
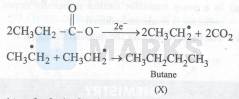
At cathode (reduction):
$$
\begin{aligned}
& 2 \mathrm{H}_2 \mathrm{O}+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{OH}^{-}+2 \mathrm{H}^{+} \\
& 2 \mathrm{H}^{+} \rightarrow \mathrm{H}_2(\mathrm{Y}) \\
& 2 \mathrm{Na}^{+}+2 \mathrm{OH}^{-} \rightarrow 2 \mathrm{NaOH}
\end{aligned}
$$
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.