Search any question & find its solution
Question:
Answered & Verified by Expert
In the organic compound \(\mathrm{CH}_2=\mathrm{CH}-\mathrm{CH}_2-\mathrm{CH}_2-\) \(\mathrm{C} \equiv \mathrm{CH}\), the \(\mathrm{C}_2-\mathrm{C}_3\) bond is the pair of hybridised orbitals involved in the formation of
(a) \(s p-s p^2\)
(b) \(s p-s p^3\)
(c) \(s p^2-s p^3\)
(d) \(s p^3-s p^3\)
(a) \(s p-s p^2\)
(b) \(s p-s p^3\)
(c) \(s p^2-s p^3\)
(d) \(s p^3-s p^3\)
Solution:
2355 Upvotes
Verified Answer
(c) When both double and triple bonds are present, double bond is given preference while numbering the carbon chain. Thus,
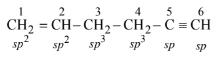
\(\therefore \quad \mathrm{C}_2-\mathrm{C}_3\) bond is formed by overlap of \(s p^2-s p^3\) orbital.
Thus, option (c) is correct.
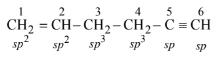
\(\therefore \quad \mathrm{C}_2-\mathrm{C}_3\) bond is formed by overlap of \(s p^2-s p^3\) orbital.
Thus, option (c) is correct.
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.