Search any question & find its solution
Question:
Answered & Verified by Expert
In the reaction, $\mathrm{P}+\mathrm{Q} \longrightarrow ? \mathrm{R}+\mathrm{S}$
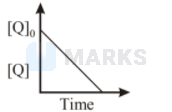
The time taken for $75 \%$ reaction of $P$ is twice the time taken for $50 \%$ reaction of $\mathrm{P}$. The concentration of $\mathrm{Q}$ varies with reaction time as shown in the figure. The overall order of the reaction is
Options:
The time taken for $75 \%$ reaction of $P$ is twice the time taken for $50 \%$ reaction of $\mathrm{P}$. The concentration of $\mathrm{Q}$ varies with reaction time as shown in the figure. The overall order of the reaction is

Solution:
1520 Upvotes
Verified Answer
The correct answer is:
1
For $\mathrm{P}$, if $\mathrm{t}_{50 \%}=\mathrm{x}$ then $\mathrm{t}_{75 \%}=2 \mathrm{x}$
This is true only for first order reaction. So, order with respect to $\mathrm{P}$ is $1 .$ Further the graph shows that concentration of $\mathrm{Q}$ decreases with time. So rate, with respect to $\mathrm{Q},$ remains constant. Hence, it is zero order wrt Q. So, overall order is $1+0=1$
This is true only for first order reaction. So, order with respect to $\mathrm{P}$ is $1 .$ Further the graph shows that concentration of $\mathrm{Q}$ decreases with time. So rate, with respect to $\mathrm{Q},$ remains constant. Hence, it is zero order wrt Q. So, overall order is $1+0=1$
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.