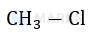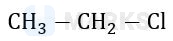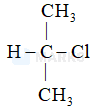Search any question & find its solution
Question:
Answered & Verified by Expert
In which of the following options chlorine will act as the best leaving group.
Options:
Solution:
2635 Upvotes
Verified Answer
The correct answer is:
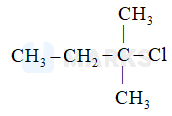
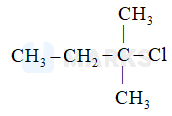
The chlorine atom that leaves to give us the most stable carbocation will act as the best leaving group. Among the given options, the Cl attached to a 3o carbon will act as the best leaving group as this would give a stable 3o carbocation (stable due to highest extent of hyperconjugation).
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.