Search any question & find its solution
Question:
Answered & Verified by Expert
In which of the following reactions, $t$-butyl benzene is formed
1. Benzene $+t$-butyl chloride $\stackrel{\mathrm{AlCl}_3}{\longrightarrow}$
2. Benzene $+\left(\mathrm{CH}_3\right)_2 \mathrm{C}=\mathrm{CH}_2 \stackrel{\mathrm{BF}_3}{\stackrel{\mathrm{HF}}{\longrightarrow}}$
3. Benzene $+t$-butyl alcohol $\stackrel{\mathrm{H}_2 \mathrm{SO}_4}{\longrightarrow}$
4. Benzene + butanoyl chloride $\underset{\mathrm{Zn} / \mathrm{HCl}}{\stackrel{\mathrm{AlCl}_3}{\longrightarrow}}$
Options:
1. Benzene $+t$-butyl chloride $\stackrel{\mathrm{AlCl}_3}{\longrightarrow}$
2. Benzene $+\left(\mathrm{CH}_3\right)_2 \mathrm{C}=\mathrm{CH}_2 \stackrel{\mathrm{BF}_3}{\stackrel{\mathrm{HF}}{\longrightarrow}}$
3. Benzene $+t$-butyl alcohol $\stackrel{\mathrm{H}_2 \mathrm{SO}_4}{\longrightarrow}$
4. Benzene + butanoyl chloride $\underset{\mathrm{Zn} / \mathrm{HCl}}{\stackrel{\mathrm{AlCl}_3}{\longrightarrow}}$
Solution:
2992 Upvotes
Verified Answer
The correct answer is:
1, 2 and 3
The given reactions take place as follows.
1. The replacement of H-atom in benzene, by alkyl group can be brought about alkyl chloride in presence of anhydrous $\mathrm{AlCl}_3$. Reaction is known as Friedel-Craft's alkylation reaction.
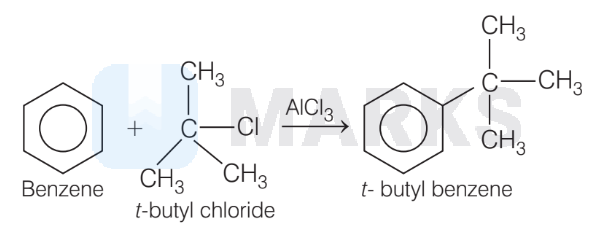
2. In this reaction, benzene reacts to 2 -methyl propane, produce $t$-butyl benzene.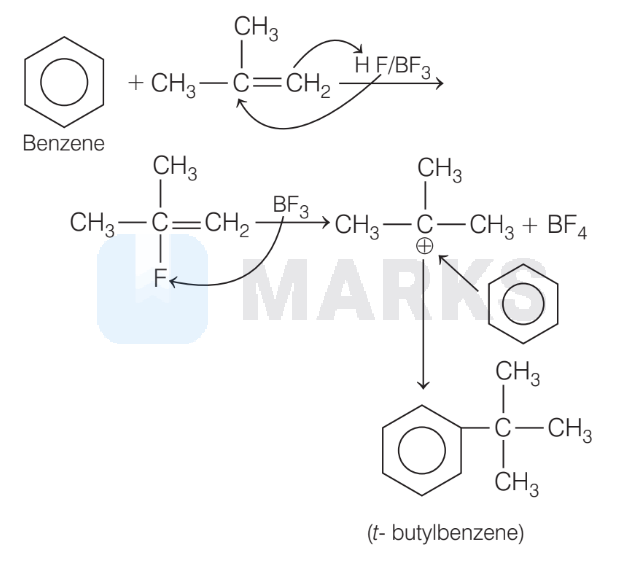
3. This can also be carried out by using alkanol and $\mathrm{H}_2 \mathrm{SO}_4$.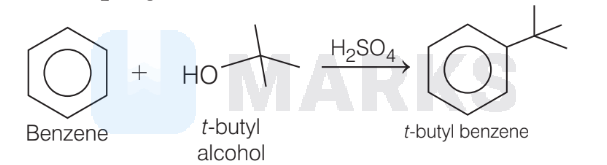
4. The reaction I, II and III gives $t$-butyl benzene, while IV gives phenyl propanone.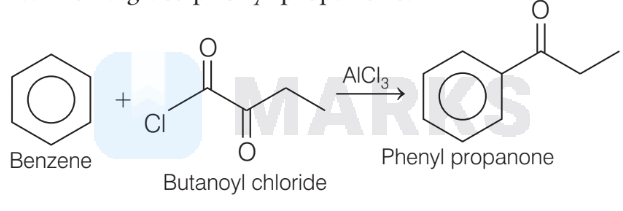
1. The replacement of H-atom in benzene, by alkyl group can be brought about alkyl chloride in presence of anhydrous $\mathrm{AlCl}_3$. Reaction is known as Friedel-Craft's alkylation reaction.

2. In this reaction, benzene reacts to 2 -methyl propane, produce $t$-butyl benzene.

3. This can also be carried out by using alkanol and $\mathrm{H}_2 \mathrm{SO}_4$.

4. The reaction I, II and III gives $t$-butyl benzene, while IV gives phenyl propanone.

Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.