Search any question & find its solution
Question:
Answered & Verified by Expert
Is boric acid a protic acid ? Explain.
Solution:
2303 Upvotes
Verified Answer
It is a not a protic acid since it does not ionize in \(\mathrm{H}_2 \mathrm{O}\) to give a proton:
\(\mathrm{H}_3 \mathrm{BO}_3+\mathrm{H}_2 \mathrm{O} \rightleftharpoons \mathrm{H}_2 \mathrm{BO}_3^{-}+\mathrm{H}_3 \mathrm{O}^{+}\)
because of the small size of boron atom and presence of only six electrons in its valence shell, \(\mathrm{B}(\mathrm{OH})_3\) accepts a lone pair of electrons from the oxygen atom of the \(\mathrm{H}_2 \mathrm{O}\) molecule to form a hydrated species.
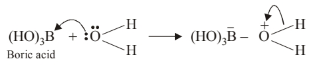
The +ve charge on the \(\mathrm{O}\)-atom, in turn, pulls the \(\sigma\) electrons of the \(\mathrm{O}-\mathrm{H}\) bond towards itself thereby facilitating the release of a proton. As a result, \(\mathrm{B}(\mathrm{OH})_3\) acts as a weak monobasic Lewis acid and thus reacts with \(\mathrm{NaOH}\) solution to form sodium metaborate.
\(\mathrm{B}(\mathrm{OH})_3+\mathrm{NaOH} \longrightarrow \underset{\text{Sod metaborate}}{\mathrm{Na}^{+}\left[\mathrm{B}(\mathrm{OH})_4\right]^{-}}\)
\(\mathrm{H}_3 \mathrm{BO}_3+\mathrm{H}_2 \mathrm{O} \rightleftharpoons \mathrm{H}_2 \mathrm{BO}_3^{-}+\mathrm{H}_3 \mathrm{O}^{+}\)
because of the small size of boron atom and presence of only six electrons in its valence shell, \(\mathrm{B}(\mathrm{OH})_3\) accepts a lone pair of electrons from the oxygen atom of the \(\mathrm{H}_2 \mathrm{O}\) molecule to form a hydrated species.

The +ve charge on the \(\mathrm{O}\)-atom, in turn, pulls the \(\sigma\) electrons of the \(\mathrm{O}-\mathrm{H}\) bond towards itself thereby facilitating the release of a proton. As a result, \(\mathrm{B}(\mathrm{OH})_3\) acts as a weak monobasic Lewis acid and thus reacts with \(\mathrm{NaOH}\) solution to form sodium metaborate.
\(\mathrm{B}(\mathrm{OH})_3+\mathrm{NaOH} \longrightarrow \underset{\text{Sod metaborate}}{\mathrm{Na}^{+}\left[\mathrm{B}(\mathrm{OH})_4\right]^{-}}\)
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.