Search any question & find its solution
Question:
Answered & Verified by Expert
$K_a$ for $\mathrm{HCN}$ is $5 \times 10^{-10}$ at $25^{\circ} \mathrm{C}$. For maintaining a constant $\mathrm{pH}=9$, the volume of $5 \mathrm{M} \mathrm{KCN}$ solution required to be added to $10 \mathrm{~mL}$ of $2 \mathrm{M} \mathrm{HCN}$ solution is
Options:
Solution:
1946 Upvotes
Verified Answer
The correct answer is:
$2 \mathrm{~mL}$
$\mathrm{pH}=\mathrm{p} K_a+\log \frac{[\text { Salt }]}{[\text { Acid }]}$
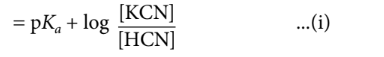
Let the volume of $\mathrm{KCN}$ solution required be $V \mathrm{~mL}$
$\therefore \quad[\mathrm{KCN}]=\frac{5 \times V}{V+10}$ and $[\mathrm{HCN}]=\frac{10 \times 2}{V+10}$
Now from eqn. (i),
$\begin{aligned} & \mathrm{pH}=-\log \left(5 \times 10^{-10}\right)+\log \left[\frac{5 \times V}{V+10} / \frac{10 \times 2}{V+10}\right] \\ & 9=-\log \left(5 \times 10^{-10}\right)+\log \frac{V}{4}\end{aligned}$
On solving, $V=1.99 \approx 2 \mathrm{~mL}$
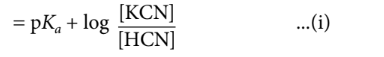
Let the volume of $\mathrm{KCN}$ solution required be $V \mathrm{~mL}$
$\therefore \quad[\mathrm{KCN}]=\frac{5 \times V}{V+10}$ and $[\mathrm{HCN}]=\frac{10 \times 2}{V+10}$
Now from eqn. (i),
$\begin{aligned} & \mathrm{pH}=-\log \left(5 \times 10^{-10}\right)+\log \left[\frac{5 \times V}{V+10} / \frac{10 \times 2}{V+10}\right] \\ & 9=-\log \left(5 \times 10^{-10}\right)+\log \frac{V}{4}\end{aligned}$
On solving, $V=1.99 \approx 2 \mathrm{~mL}$
Looking for more such questions to practice?
Download the MARKS App - The ultimate prep app for IIT JEE & NEET with chapter-wise PYQs, revision notes, formula sheets, custom tests & much more.